By: Teah Jazey, Senior Product Safety & Regulatory Affairs Specialist, email
In July 2024 the Government of Canada (Health Canada) published a Notice with respect to certain per- and polyfluoroalkyl substances (PFAS). This is a mandatory information-gathering Notice that was published in the Canada Gazette Part 1. Under the Canadian Environmental Protection Act, 1999, Section 71, you are required to submit data if the Notice applies to you. This particular Notice lists 312 substances that the Government is seeking more information on, specifically about PFAS. The list of substances can be found in the Canada Gazette Part 1, published July 27 2024. Find the link here.
Parties to Respond
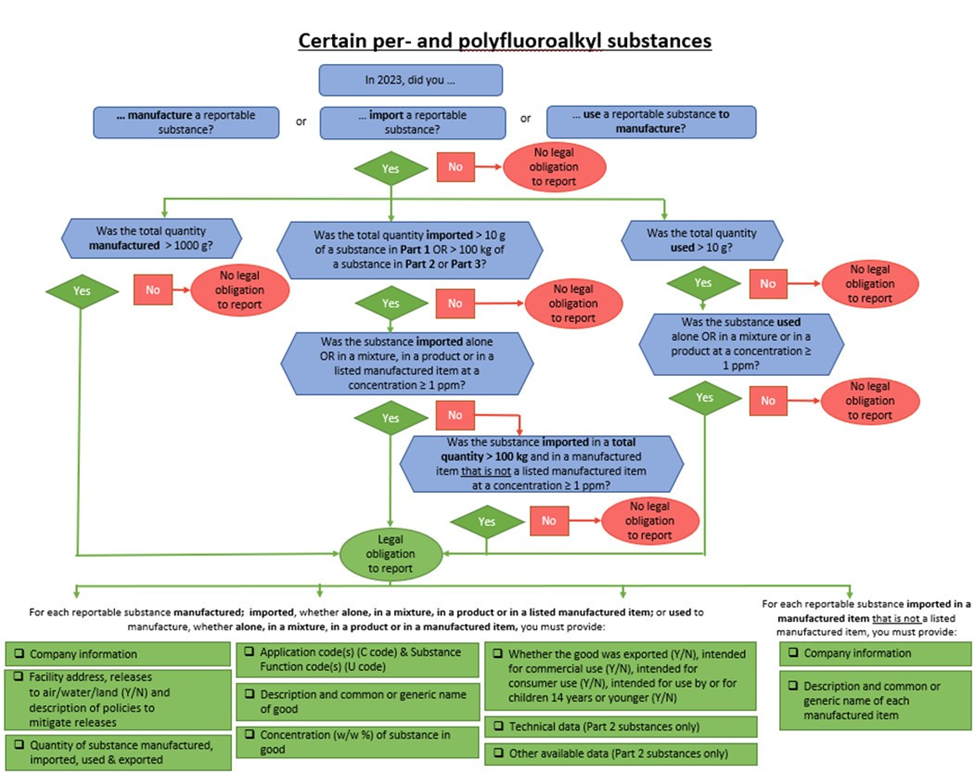
This Notice will apply to anyone who has participated in the following activities during the 2023 calendar year:
- Manufactured a total quantity greater than 1000 g of a substance listed in Schedule 1.
- Imported a total quantity greater than 10 g of a substance listed in Part 1 of Schedule 1, OR a total quantity greater than 100 kg of a substance listed in Part 2 or Part 3 of Schedule 1, whether the substance was alone, or at a concentration equal to or above 1 ppm in a mixture or in a product or at a concentration equal to or above 1 ppm in one of the categories of manufactured items
- Imported a total quantity greater than 100 kg of any substance listed in Schedule 1 at a concentration equal to or above 1 ppm in a manufactured item NOT listed in the categories of manufactured items
- Used total quantities greater than 10 g of a substance listed in Schedule 1, whether the substance was alone, or at a concentration equal to or above 1 ppm in a mixture or in a product, in the manufacture of a mixture, a product or a manufactured item
Even if you do not meet the Notice requirements but are involved with either listed or non-listed PFAS, you are strongly encouraged to submit a Declaration of Non-Engagement to substances@ec.gc.ca. Be sure to include your mailing and contact information in your response. The email subject line must also include “PFAS DNE.” Sample declarations can be found in the Guidance Document.
How to Respond to the Notice
If you meet the criteria listed above, then you must submit a Section 71 response using the Excel Reporting File (ERF) found here. Below we have also included a link to the Guidance Document which shows various examples and descriptions on how to fill out the Reporting form. The Excel File itself also contains an instructions tab with information on how to complete the form. Once completed, the ERF should be submitted via Environment Canada’s Single Window online reporting system.
Deadline to Report
Please note that the deadline to provide information and submit a notice is January 29 2025. If you require an extension, you must request this prior to the January 29th deadline.
Helpful Resources:
- Guidance Document: https://www.canada.ca/en/environment-climate-change/services/evaluating-existing-substances/pfas-s71-guidance-manual.html
- Responding to the PFAS Notice: https://www.canada.ca/en/environment-climate-change/services/evaluating-existing-substances/pfas-notice-reporting-form.html
- Canada Gazette Part 1 – July 27 2024 – Schedule 1 (Page 78/177): https://www.gazette.gc.ca/rp-pr/p1/2024/2024-07-27/pdf/g1-15830.pdf
Reporting Criteria and Requirements Flowchart
Talk to Our Experts
Learn more about our services on our service page.
DELL TECH HAS PROVIDED PROFESSIONAL, CONFIDENTIAL CONSULTING SERVICES TO THE SPECIALTY CHEMICAL INDUSTRY IN CANADA, THE USA, EUROPE AND ASIA FOR THE LAST 40 YEARS.
[INSERT_ELEMENTOR id=5705]




