SDS Expiry Date and Risk of Compliance
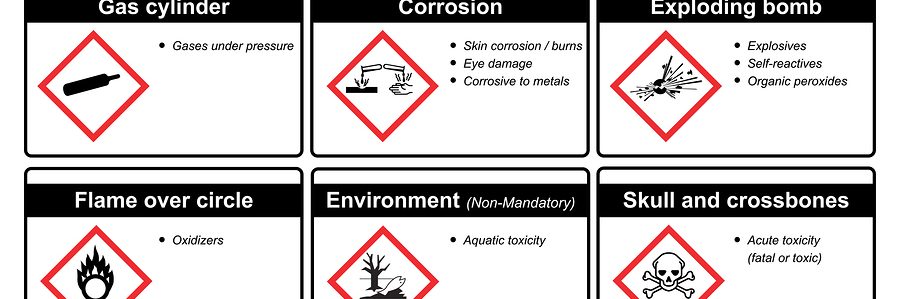
By: Alison Senyi, SENIOR PRODUCT SAFETY SPECIALIST, email Upcoming regulatory changes In January 2023, Canada published the revisions to the Hazardous Products Regulations (HPR). The document reflects the HPR requirements…