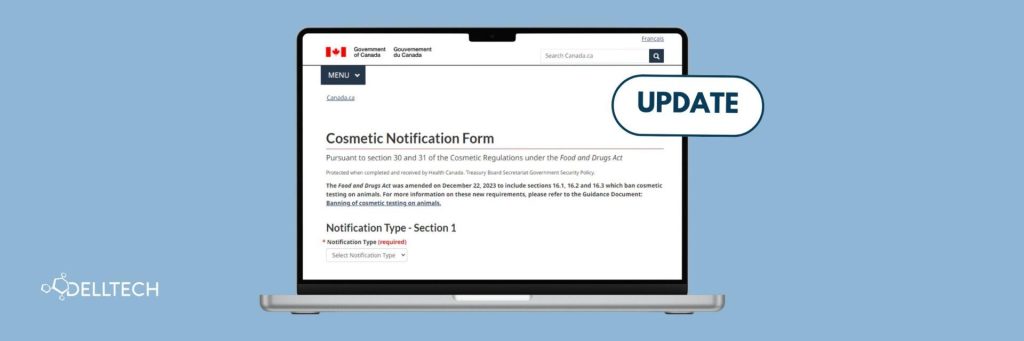
Updates to Canada’s Cosmetic Notification Form: Key Changes and Compliance Tips
By: Ivy Tang, PRINCIPAL REGULATORY CONSULTANT, email The Cosmetic Notification Form (CNF) is a regulatory requirement in Canada designed to ensure that cosmetics comply with safety standards outlined in…