By: Teah Jazey, Regulatory Affairs and Product Safety Associate, email
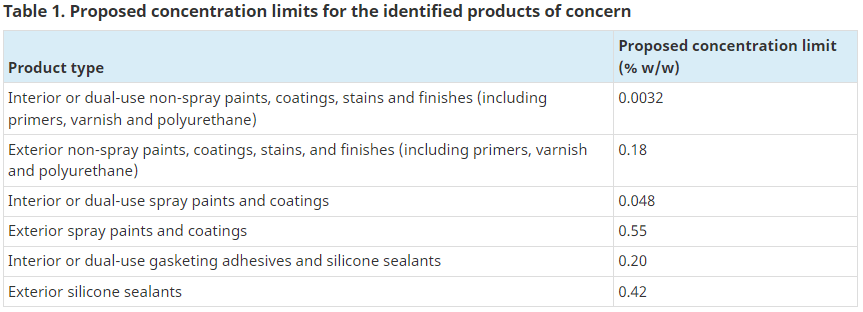
On July 15, 2022, Health Canada published a consultation document on proposed new risk management (RM) actions for 2-butanone, oxime, commonly known as methyl ethyl ketoxime (MEKO). MEKO is a widely used anti-skinning agent in the formulation of paints, varnishes, stains, finishes, coatings, adhesives, and sealants. The proposed risk management includes new concentration limits in response to a Performance Measurement Evaluation published in July 2020 that determined the existing voluntary Code of Practice for paint and coatings had not achieved its objective. Given the lack of adoption of voluntary measures, the Government of Canada is proposing a new regulatory approach to risk management that expands on the Code of Practice, including new concentration limits proposed for MEKO in sealants and adhesives (see Table 1).
The Government of Canada is looking for more information on MEKO’s use in consumer products, potential alternatives, and the costs associated with compliance with the proposed changes. If you or your company would like to submit comments, you must do so prior to October 13 2022 as this is when the consultation period ends.
To submit your comments email Andrew Beck at chemicalsubstanceschimiques@hc-sc.gc.ca with the subject line: “Consultation Document on Proposed New Risk Management Actions for Butanone Oxime”.
Contact:
Dell Tech
Teah Jazey
Regulatory Affairs and Product Safety Associate
519-858-5021
teah@delltech.com
Dell Tech has provided professional, confidential consulting services to the chemical specialty
industry in Canada, the USA, Europe, and Asia for the last 40 years.
Contact us today for more information.
www.delltech.com