By: Kirsten Alcock, Manager of Product Safety, email
The consumer packaging and labeling regulations advise on the requirements for what is needed on your label IN ADDITION to other requirements that may be required such as the CCCR, 2001. For example, if your product is hazardous by the CCCR criteria, (Consumer Chemicals and Containers Regulations, 2001), your package must have all the requirements for the CCCR category your product falls under as well as the packaging and labeling requirements.
Requirements under the Consumer Packaging and Labelling Regulations include but are not limited to:
- Net quantity declaration. When you are selling your product to Canada, the net quantity must be metric.
- Metric abbreviations. Ensure they are those that are accepted by the Government of Canada.
- Bilingualism. The official languages of Canada are English AND Canadian French. You must ensure that the French you are using is Canadian French.
- Requirements for Principal Display Panel.
One of the biggest errors I see is how the net quantity is displayed onto Canadian labels. The spelling here for some words is different than within the United States. Here is a little cheat sheet for you to use if you sell your consumer product in Canada:
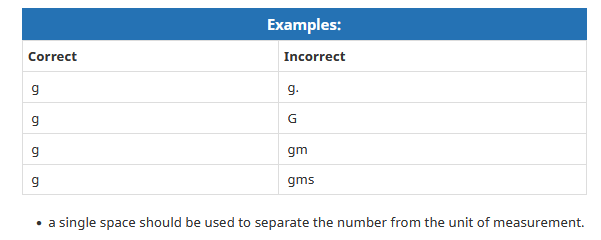
 Here is another table that shows how NOT to display your abbreviation.
Here is another table that shows how NOT to display your abbreviation.
Contact us for further information on how we can help you meet your label compliance for Canada. We have the experience to know what Health Canada is looking for. We can also help by providing properly translated labels into Canadian French.
Contact:
Dell Tech
Kirsten Alcock, B.Sc. (Hons)
Manager, Product Safety Group
519-858-5074
kirsten@delltech.com
Dell Tech has provided professional, confidential consulting services to the chemical specialty
industry in Canada, the USA, Europe, and Asia for the last 40 years.
Contact us today for more information.