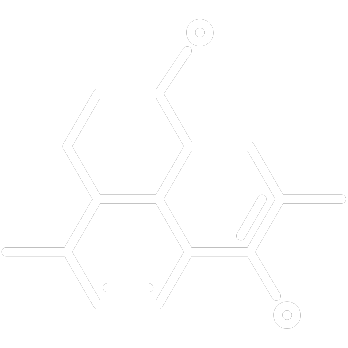
Formula Assessment
We examine your product’s ingredients and dosage to determine if the formulation meets Canadian regulatory requirements. Our team identifies any compliance concerns and flags substances that may require additional justification or are not permitted.