Good Manufacturing Practices
are internationally recognized standards that ensure drugs and natural health products meet the appropriate quality and safety standards for their intended use prior to being sold.
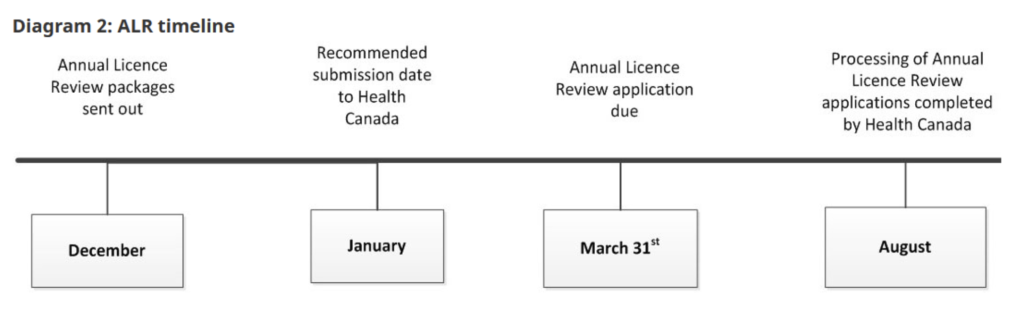
Health Canada inspects establishments that fabricate, package, label, test, import, distribute or wholesale drugs and for some licensable activities active pharmaceutical ingredients (API). GMPs are a requirement under Division 2 of the Food and Drug Regulations.